New Publication in the HIPOBAT project
Title
Impurities in Na2S Precursor and Their Effect on the Synthesis of W-Substituted Na3PS4: Enabling 20 mS cm−1 Thiophosphate Electrolytes for Sodium Solid-State Batteries
Link
https://doi.org/10.1002/aenm.202503047
Summary
Sodium solid-state batteries are intensively researched, expecting a resource-uncritical alternative to their lithium counterparts. As in the case of lithium, sulfide-type electrolytes show promising ionic conductivities 𝝈, and Na3PS4-type solid electrolytes are intensively investigated. Aliovalent substitution of P5+ by W6+ is shown to achieve a sodium ion conductivity 𝝈(Na+) beyond 10 mS cm−1, rendering them good candidates for cathode composites.
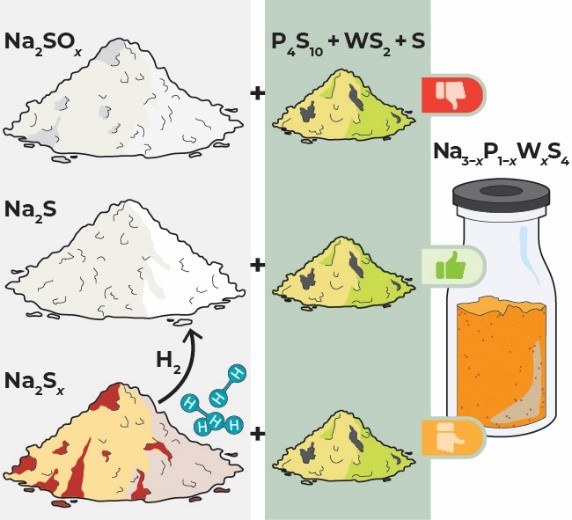
Yet, incorporating WS42− into the crystal lattice of Na3PS4 is deemed challenging, and Na3−xP1−xWxS4 electrolytes suffer from WS2 residue after synthesis. In this work, impurities in the precursor Na2S are identified and the detrimental influence of SOx groups in Na2S on the synthesis of Na3PS4 and Na3−xP1−xWxS4 is demonstrated. The behavior of oxygen as impurity during synthesis is pinpointed, and complete incorporation of tungsten up to x ≈ 0.25 in Na3−xP1−xWxS4 by purified Na2S is achieved, realizing up to 𝝈(Na+) = 26.4 mS cm−1 at room temperature.
Interview with the lead author and researcher in the HIPOBAT project, Felix Schnaubelt, JLU Giessen
1. Your work comprehensively describes the purification of commercially available Na2S precursor for the synthesis of thiophosphate electrolytes. What was the initial motivation to look into this in more detail?
The goal of HiPoBat is to investigate and develop high power sodium batteries and Na3−xP1−xWxS4 fulfills the σion >10 mS cm−1 requirement for high power solid-state batteries with sufficiently thick cathode composites. Thus, I attempted to synthesize Na2.9P0.9W0.1S4 but could not obtain the desired phase. The presence of oxygen-containing phases in the product mixture quickly led to the assumption of contaminations in the precursor(s) since the synthesis was conducted in an inert atmosphere. Analyzing the precursors revealed significant Na2SOx contamination in the only commercially available anhydrous Na2S at that time. This was the reason I investigated purification and synthesis methods to obtain clean Na2S. I quickly decided that the H2 purification route was most suitable for our synthesis capabilities in our laboratory.
2. What was the most difficult experimental obstacle to overcome to receive highly pure Na2S (e.g., recontamination)?
The most difficult obstacle was to find the right H2 concentration and reaction temperature. I started with a relatively low H2 content of 5 % (95 % Ar) and a low temperature of 450 °C, like the literature suggested, but found that these conditions do not work in our oven setup. I gradually increased the temperature and H2 content until pure Na2S was obtained. In our setup, I settled with a pure H2 atmosphere and a temperature of 650 °C. Luckly, recontamination does not seem to occur when the purified Na2S has very brief atmosphere contact. We could not detect any Na2SOx species with XPS, a relatively sensitive method for surface contaminations.
3. On what scale (weight) did you carry out the purification steps? Did you have to construct special sealed containers to move the material or special accessories for the furnace?
I conduct the H2 treatment with a batch size of 10-12 g Na2S. I did not design any special containers and found creative use of existing equipment. Any air-tight container that can be evacuated is sufficient to transfer the purified Na2S to a glovebox.
4. In the second part of your publication, it is described how the high purity of Na2S enables the synthesis phase pure P5+ and W6+ tungsten substituted Na3PS4-type solid electrolytes with very high Na+ conductivity of 20 mS cm-1. Have you tested this electrolyte already in half or full electrochemical cells? What are the remaining challenges in terms of electrochemical stability and compatibility?
Anodic and cathodic stability remain a great challenge. We conducted preliminary coulometric titration time analysis (CTTA) measurements and observed that tungsten substitution decreases stability against Na metal drastically. Prior work from our group demonstrated severe degradation in cathode composites containing sulfide-based electrolytes as well. Increasing the stability of Na3−xP1−xWxS4 electrolytes against active materials is something we are actively looking into.

